The Vogel Laboratory: Intracellular Cargo Transport in Congenital Intestinal Diseases
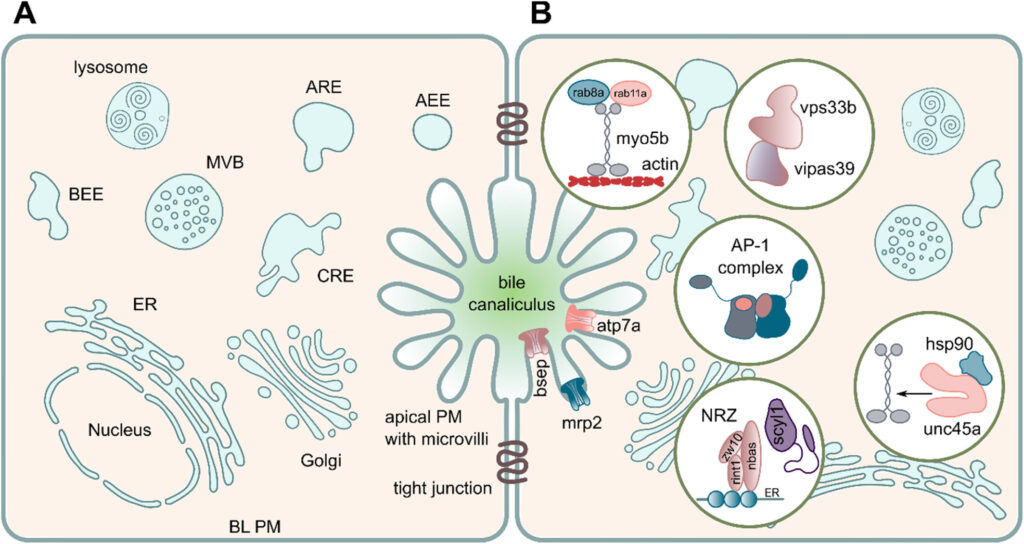
We are focusing on congenital intestinal and liver diseases, which are often caused by mutations in genes that are essential for correct intestinal-epithelial polarization. For example, we have identified mutations in MYO5B and STX3 in microvillus inclusion disease: both genes are essential for the polarized apical cargo transport of transmembrane transporters (Vogel GF et al., JCB, 2015; Vogel GF et al., JCI Insight, 2017; Szabó L et al., Traffic, 2024). We are now turning our attention to myo5b-associated cholestatic liver disease and myo5b-independent apical transport in intestinal epithelial cells.
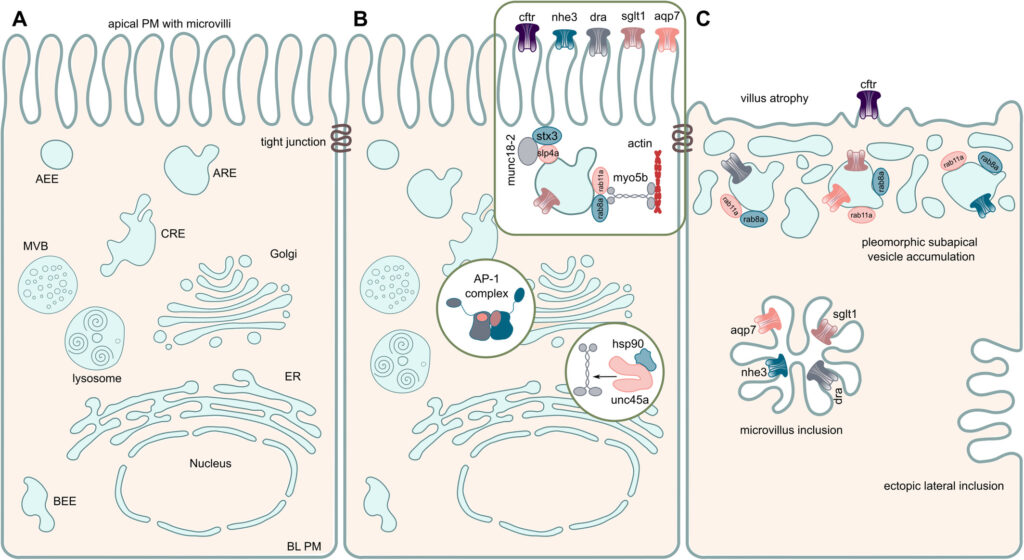
Moreover, we are interested in describing novel genetic variants associated with congenital enteropathies or hepatopathies. We recently described the first case of a congenital enteropathy due to mutations in ezrin (Vogel GF et al., Human Genetics, 2025), a protein involved in the establishment of proper apical polarity and plasma membrane composition in epithelial cells, as well as the first cases of acute liver failure associated with variants in the TRPM7 gene (Schlieben LD et al., Hepatology Communications 2024).
We are also addressing the role of the retriever complex and the poorly characterized protein TM9SF4 in polarized plasma membrane cargo trafficking in epithelial cells.
Furthermore, we study the liver immunesystem associated with acute hepatitis of unknown origin (Röttele F et al., Gut 2025) and autoimmune liver disease, the enterohepatic circulation in patients with progressive familial intrahepatic cholestasis (PFIC1), and the role of the gut microbiome in association with gut and hepatic diseases (Zöggeler T et al., Gut Microbes 2025; Aldrian D et al., Inflammatory Bowel Diseases 2025).
Publications
Meta-analysis of shotgun sequencing of gut microbiota in obese children with MASLD or MASH. Zöggeler T, Kavallar AM, Pollio AR, Aldrian D, Decristoforo C, Scholl-Bürgi S, Müller T, Vogel GF.Gut Microbes. 2025 Dec;17(1):2508951. doi: 10.1080/19490976.2025.2508951. Epub 2025 May 21.
Characteristic immune cell interactions in livers of children with acute hepatitis revealed by spatial single-cell analysis identify a possible postacute sequel of COVID-19.Röttele F, Zollner A, Mogler C, Yuksel M, Arikan C, Karl V, Aberle JH, Aberle SW, Kogler H, Vécsei A, Vodopiutz J, Salié H, Gräser A, Krimmel L, Martin P, Lurz E, Maier FI, Woelfle L, Nobre S, Goncalves I, Kern L, Schwemmle M, Boettler T, Hofmann M, Hasselblatt P, Thimme R, Tilg H, Müller T, Vogel GF, Bengsch B.Gut. 2025 Apr 29:gutjnl-2024-333880. doi: 10.1136/gutjnl-2024-333880. Online ahead of print.
Congenital enteropathy caused by ezrin deficiency. Vogel GF, Klee KMC, Demir AM, Garczarczyk-Asim D, Hess MW, Huber LA, Müller T, Janecke AR.Hum Genet. 2025 May;144(5):505-514. doi: 10.1007/s00439-025-02738-w. Epub 2025 Mar 26.
Missense variants in the TRPM7 α-kinase domain are associated with recurrent pediatric acute liver failure. Schlieben LD, Achleitner MT, Bourke B, Diesner M, Feichtinger RG, Fichtner A, Flechtenmacher C, Hadzic N, Hegarty R, Heilos A, Janecke A, Konstantopoulou V, Lenz D, Mayr JA, Müller T, Prokisch H, Vogel GF. Hepatol Commun. 2024 Nov 29;8(12):e0598. doi: 10.1097/HC9.0000000000000598. eCollection 2024 Dec 1.
Intracellular Trafficking Defects in Congenital Intestinal and Hepatic Diseases. Szabó L, Pollio AR, Vogel GF. Traffic. 2024 Aug;25(8):e12954. doi: 10.1111/tra.12954.
Kinesin family member 12-related hepatopathy: A generally indolent disorder with elevated gamma-glutamyl-transferase activity. Vogel GF, Podpeskar A, Rieder D, Salzer H, Garczarczyk-Asim D, Wang L, Abuduxikuer K, Wang JS, Scharrer A, Faqeih EA, Aseeri AT, Vodopiutz J, Heilos A, Pichler J, Huber WD, Müller T, Knisely AS, Janecke AR. Clin Genet. 2024 Sep;106(3):224-233. doi: 10.1111/cge.14524. Epub 2024 Mar 29.
Natural history of Wolcott-Rallison syndrome: A systematic review and follow-up study. Aldrian D, Bochdansky C, Kavallar AM, Mayerhofer C, Deeb A, Habeb A, Romera Rabasa A, Khadilkar A, Uçar A, Knoppke B, Zafeiriou D, Lang-Muritano M, Miqdady M, Judmaier S, McLin V, Furdela V, Müller T, Vogel GF.
A CRISPR screen in intestinal epithelial cells identifies novel factors for polarity and apical transport. Klee KMC, Hess MW, Lohmüller M, Herzog S, Pfaller K, Müller T, Vogel GF, Huber LA. Elife. 2023 Jan 20;12:e80135. doi: 10.7554/eLife.80135. PMID: 36661306.
Collaborations
Andreas Janecke, Department of Paediatrics I, Medical University of Innsbruck, Austria
Bertram Bengsch, Department of Internal Medicine II, Freiburg University Medical Center, Germany
Dominic Lenz, Department of Pediatrics, Medical University of Heidelberg, Germany
Saskia Wortmann, Paediatrics, University Hospital Salzburg, Austria
Funding
ÖNB Anniversary Fund 18019, TWF Grant 0404/2386, FWF Grant P35805B, ÖGKJ research grant, ESPGHAN research grant, Takeda Pharmaceuticals, Ipsen Pharma, Mirum Pharmaceuticals
Contact

Priv.-Doz. Dr. med. univ. Georg-Friedrich, PhD
Email: georg.vogel@i-med.ac.at
Phone: +43 512 504 82184

Vogel-Lab 2025, from left: Beatrix, Stefan ,Georg, Luca, Doris, Adam