The Stasyk Laboratory: Molecular architecture of native lysosomal protein assemblies
Lysosomes are intracellular signaling platforms that govern cell growth, division and differentiation, and provide a hub for anabolic and catabolic signaling. Despite great progress in our understanding of the molecular mechanisms of sensing such important nutrients as amino acids, cholesterol and glucose, key questions on how components of sensing and signaling machineries work together remain pending. The LAMTOR/Ragulator complex is a key regulator of lysosomal mTORC1, MAPK and AMPK signaling. LAMTOR also coordinates lysosome biogenesis via the BORC complex. The presence of distinct LAMTOR complexes on lysosomes raises fundamental questions on how anabolic and catabolic signaling, lysosomal biogenesis and positioning are coordinated on the mechanistic level to coordinate cellular fitness.
We aim to identify all LAMTOR and BORC associated protein assemblies on intact lysosomes under different growth factors and nutrition conditions and obtain evidence for direct and endogenous protein-protein interactions. We are applying cross-linking mass spectrometry (XL-MS) in combination with sophisticated subcellular fractionation to understand the molecular architectures of the different LAMTOR and BORC assemblies (super-complexes) at endogenous levels and under different physiological conditions. A unique advantage of this approach is that native endogenous protein interactions are captured using a chemical cross-linker reactive towards specific amino acid side-chains that are in close spatial proximity. In a parallel approach, enriched lysosomes are first cross-linked followed by affinity purification of protein complexes to reveal signaling-dependent rearrangements.
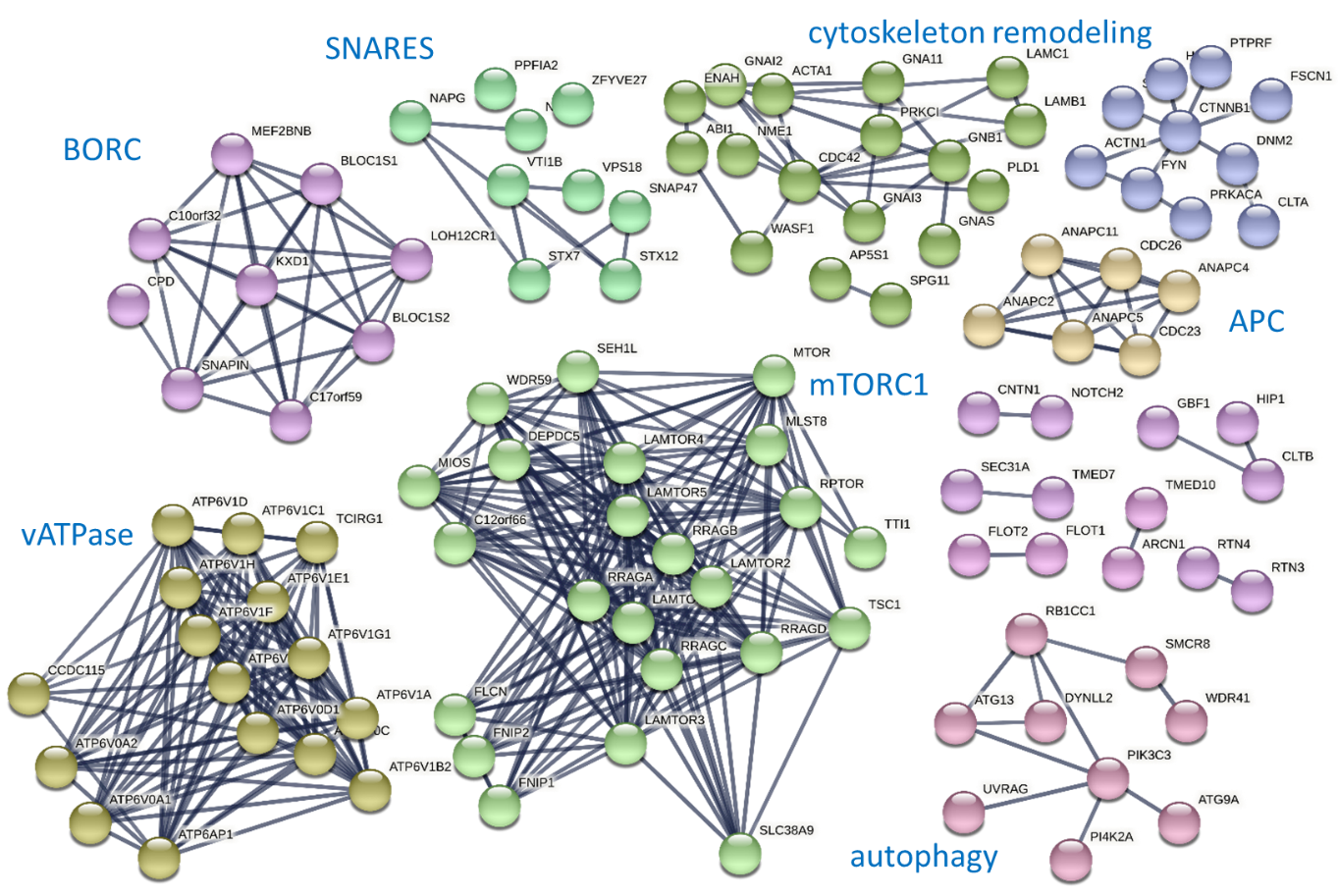
Molecular mechanisms of how LAMTOR relates to different sensing and signaling protein machines, including BORC in particular, and how these interactions coordinate lysosomal biogenesis and function, to maintain cellular homeostasis, are largely unknown and are thus the focus of our interest.
Publications
LAMTOR/Ragulator regulates lipid metabolism in macrophages and foam cell differentiation. Lamberti G, De Smet CH, Angelova M, Kremser L, Taub N, Herrmann C, Hess MW, Rainer J, Tancevski I, Schweigreiter R, Kofler R, Schmiedinger T, Vietor I, Trajanoski Z, Ejsing CS, Lindner HH, Huber L, Stasyk T. FEBS Lett. 2020; 594(1):31-42. doi: 10.1002/1873-3468.13579
Biogenesis of lysosome-related organelles complex-1 (BORC) regulates late endosomal/lysosomal size through PIKfyve-dependent phosphatidylinositol-3,5-bisphosphate. Yordanov TE, Hipolito VEB, Liebscher G, Vogel GF, Stasyk T, Herrmann C, Geley S, Teis D, Botelho RJ, Hess MW, Huber LA. Traffic. 2019; 20(9):674-696. doi: 10.1111/tra.12679
LAMTOR/Ragulator is a negative regulator of Arl8b- and BORC-dependent late endosomal positioning. Filipek PA, de Araujo MEG, Vogel GF, De Smet CH, Eberharter D, Rebsamen M, Rudashevskaya EL, Kremser L, Yordanov T, Tschaikner P, Fürnrohr BG, Lechner S, Dunzendorfer-Matt T, Scheffzek K, Bennett KL, Superti-Furga G, Lindner HH, Stasyk T*, Huber LA*. J Cell Biol. 2017; 216(12):4199-4215. doi: 10.1083/jcb.201703061, *equal contribution as corresponding authors
Crystal structure of the human lysosomal mTORC1 scaffold complex and its impact on signaling. de Araujo MEG, Naschberger A, Fürnrohr BG, Stasyk T, Dunzendorfer-Matt T, Lechner S, Welti S, Kremser L, Shivalingaiah G, Offterdinger M, Lindner HH, Huber LA, Scheffzek K. Science. 2017 20;358(6361):377-381. doi: 10.1126/science.aao1583
SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Rebsamen M, Pochini L, Stasyk T, de Araújo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, Scorzoni S, Filipek PA, Huber KV, Bigenzahn JW, Heinz LX, Kraft C, Bennett KL, Indiveri C, Huber LA, Superti-Furga G. Nature. 2015, 519(7544):477-8,1 doi: 10.1038/nature14107
Funding
FWF P36975
Team and Contact

Dr. Taras Stasyk
Email:
taras.stasyk@i-med.ac.at
Phone: +43512900370340
Name
Taras Stasyk
Eva Rauch
Martina Höllwarth
Position
Group leader
PhD student
PhD student
