The Huber Laboratory: Lysosomal mTOR
Signalling – The Crossroads Between Signal Transduction and Endosomal Biogenesis
Our story begins in 2003 when we transformed our understanding of cellular signalling by identifying the LAMTOR/Ragulator complex and showing how the p14-MP1 scaffold anchors MAPK signalling to endosomes, a crucial step in regulating cell growth and survival (Teis et al., Dev Cell 2003). In 2007, we linked this complex to human disease, identifying p14 deficiency as the cause of a devastating primary immunodeficiency (Bohn et al, Nat Med 2007). This was the first indication that LAMTOR was more than a mere signalling hub – it is a lifeline. The plot thickened in 2015 when, in collaboration with Giulio Superti-Furga, we discovered SLC38A9, the long-sought amino-acid sensor that allows mTORC1 to ‘taste’ nutrients in lysosomes (Rebsamen et al, Nature 2015). Suddenly the pieces fell into place – LAMTOR was not only a scaffold, it was the control centre of metabolic decision-making. The structural revolution enabled us in 2017 to capture the first crystal structure of LAMTOR/Ragulator with Rag GTPases, revealing how this molecular machine aligns mTORC1 signalling on lysosomes (Araujo et al., Science 2017). In the same year, we showed that LAMTOR also controls lysosomal positioning, acting as a brake on endosomal motility (Filipek et al., JCB 2017). The story took an exciting turn in 2020, when – together with Jim Hurley and Andrea Ballabio – we linked LAMTOR dysfunction to the Birt-Hogg-Dubé syndrome, showing how faulty mTORC1 regulation drives this rare disease (Napolitano et al., Nature 2020). Finally, in 2023, we unveiled the megacomplex – a gigantic assembly of mTORC1, TFEB, Ragulator and Rags – painting a complete picture of how lysosomes orchestrate cellular metabolism (Cui et al., Nature 2023). From cancer to immune disorders, the LAMTOR complex is at the heart of disease and we are gradually unlocking its secrets, one discovery at a time.
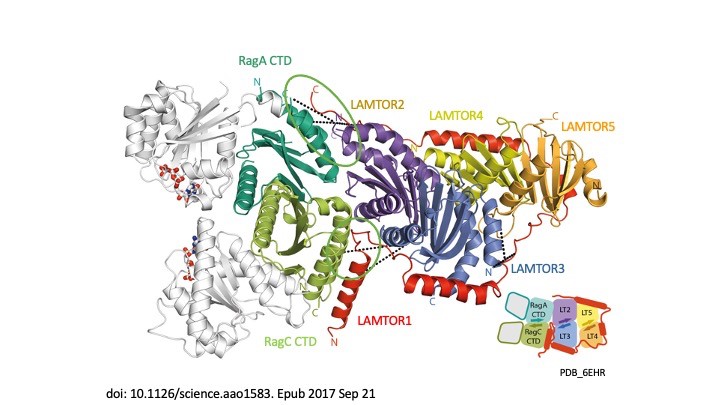
Fig. 1 The LAMTOR/Ragulator complex
Publications
- Congenital enteropathy caused by ezrin deficiency. Vogel GF, Klee KMC, Demir AM, Garczarczyk-Asim D, Hess MW, Huber LA, Mülller T, Janecke AR. Hum Genet. 2025 Mar 26. doi: 10.1007/s00439-025-02738-w. Epub ahead of print. PMID:40137958.
- Altered chaperone-nonmuscle myosin II interactions drive pathogenicity of the UNC45A c.710T>C variant in osteo-oto-hepato-enteric syndrome. Waich S, Kreidl K, Vodopiutz J, Demir AM, Pollio AR, Dostál V, Pfaller K, Parlato M, Cerf-Bensussan N, Adam R, Vogel GF, Uhlig HH, Ruemmele FM, Müller T, Hess MW, Janecke AR, Huber LA, Valovka. TJCI Insight. 2025 Mar 24;10(6):e185508. doi: 10.1172/jci.insight.185508. PMID: 40125554.
- The lysosomal LAMTOR / Ragulator complex is essential for nutrient homeostasis in brown adipose tissue. Liebscher G, Vujic N, Schreiber R, Heine M, Krebiehl C, Duta-Mare M, Lamberti G, de Smet CH, Hess MW, Eichmann TO, Hölzl S, Scheja L, Heeren J, Kratky D, Huber. LA.Mol Metab. 2023 May;71:101705. doi: 10.1016/j.molmet.2023.101705. Epub 2023 Mar 11. PMID: 36907508; PMCID: PMC10074977.
- Structure of the lysosomal mTORC1-TFEB-Rag-Ragulator megacomplex. Cui Z, Napolitano G, de Araujo MEG, Esposito A, Monfregola J, Huber LA, Ballabio A, Hurley JH.Nature. 2023 Jan 25. doi: 10.1038/s41586-022-05652-7. Online ahead of print.PMID: 36697823
- A substrate-specific mTORC1 pathway underlies Birt-Hogg-Dubé syndrome. Napolitano G, Di Malta C, Esposito A, de Araujo MEG, Pece S, Bertalot G, Matarese M, Benedetti V, Zampelli A, Stasyk T, Siciliano D, Venuta A, Cesana M, Vilardo C, Nusco E, Monfregola J, Calcagnì A, Di Fiore PP, Huber LA, Ballabio A. Nature. 2020 Sep;585(7826):597-602. doi: 10.1038/s41586-020-2444-0. Epub 2020 Jul 1. PMID: 32612235
- LAMTOR/Ragulator is a negative regulator of Arl8b- and BORC-dependent late endosomal positioning. Filipek PA, de Araujo MEG, Vogel GF, De Smet CH, Eberharter D, Rebsamen M, Rudashevskaya EL, Kremser L, Yordanov T, Tschaikner P, Fürnrohr BG, Lechner S, Dunzendorfer-Matt T, Scheffzek K, Bennett KL, Superti-Furga G, Lindner HH, Stasyk T, Huber LA. J Cell Biol. 2017 Dec 4;216(12):4199-4215. doi: 10.1083/jcb.201703061. Epub 2017 Oct 9. PMID: 28993467 Free PMC article.
- Crystal structure of the human lysosomal mTORC1 scaffold complex and its impact on signaling. de Araujo MEG, Naschberger A, Fürnrohr BG, Stasyk T, Dunzendorfer-Matt T, Lechner S, Welti S, Kremser L, Shivalingaiah G, Offterdinger M, Lindner HH, Huber LA, Scheffzek K. Science. 2017 Oct 20;358(6361):377-381. doi: 10.1126/science.aao1583. Epub 2017 Sep 21. PMID: 28935770
Collaborations
- Giulio Suerti-Furga CeMM, David Haselbach IMP, Manuela Baccarini Max Perutz Laboratories all Vienna Austria
- Dagmar Kratky Gottfried Schatz Research Center, Molecular Biology and Biochemistry, Medical University of Graz
- Andrea Ballabio TIGEM, Naples, Italy
- Structural Membrane Biology, Jinm Hurley University of California, Berkely, US
Funding
FWF P 32608 and P 26682, and FWF DOC 82 doc.fund Cellular Basis of Diseases: Molecular Control of Metabolism and Inflammation
Contact

Univ.-Prof. Dr. med.univ. Lukas A. Huber
Director
Email: Lukas.A.Huber@i-med.ac.at
Phone: +43 512 9003 70170
