The Araujo Laboratory: Lysosomal signaling complexes – Coordinating organelle function with cellular homeostasis
Lysosomes are membrane-bound organelles with several functions, from protein degradation to the control of cellular metabolism. Due to their importance for proteostasis and signaling, cells have developed mechanisms to control lysosomal numbers, size, movement, degradative capacity, interaction with other organelles and even lysophagy. Dysfunction is linked to numerous diseases, including lysosomal storage disorders, neurodegeneration, metabolic conditions and cancer.
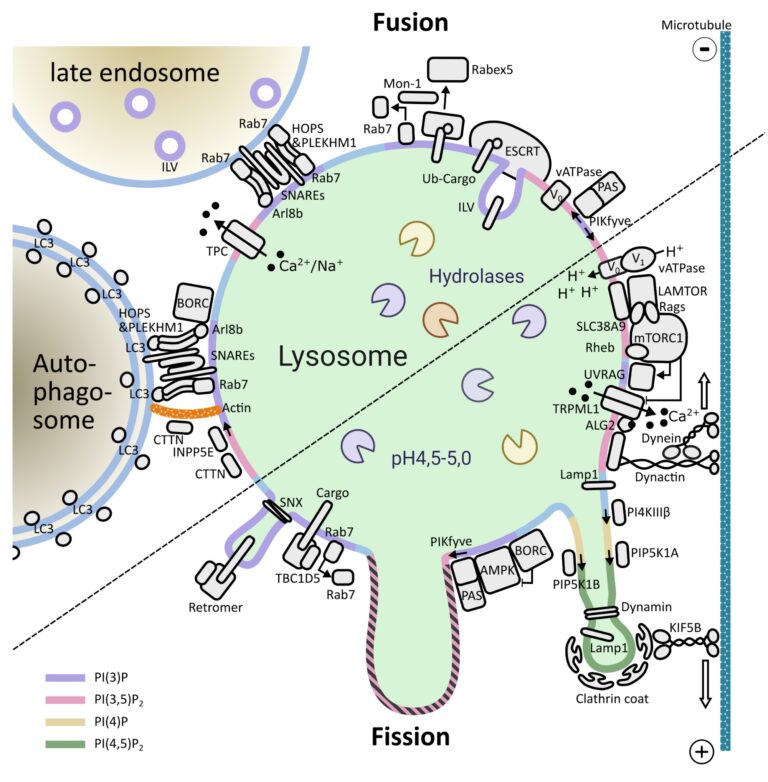
One of the key signaling complexes in the lysosomal membrane is mTORC1. Building on the knowledge from numerous laboratories over more than 30 years of research on this kinase, we are investigating how mTORC1 signaling components bind and coordinate their functions with other lysosomal proteins/complexes to maintain cellular homeostasis. We are also exploring how dysfunction of these processes contributes to disease. We use a combination of classical biochemical and cell biology methods, genome editing, live-cell imaging and super-resolution microscopy.
Publications
https://pubmed.ncbi.nlm.nih.gov/?term=de%20Araujo%20MEG&sort=date
Selected publications
Heimdörfer D, Vorleuter A, Eschlböck A, Spathopoulou A, Suarez-Cubero M, Farhan H, Reiterer V, Spanjaard M, Schaaf CP, Huber LA, Kremser L, Sarg B, Edenhofer F, Geley S, de Araujo MEG, Huettenhofer A. Truncated variants of MAGEL2 are involved in the etiologies of the Schaaf-Yang and Prader-Willi syndromes. Am J Hum Genet. 2024 Jul 11;111(7):1383-1404. doi: 10.1016/j.ajhg.2024.05.023. Epub 2024 Jun 21. PMID: 38908375
Sambri I, Ferniani M, Campostrini G, Testa M, Meraviglia V, de Araujo MEG, Dokládal L, Vilardo C, Monfregola J, Zampelli N, Vecchio Blanco FD, Torella A, Ruosi C, Fecarotta S, Parenti G, Staiano L, Bellin M, Huber LA, De Virgilio C, Trepiccione F, Nigro V, Ballabio A. . RagD auto-activating mutations impair MiT/TFE activity in kidney tubulopathy and cardiomyopathy syndrome. Nat Commun. 2023 May 15;14(1):2775. doi: 10.1038/s41467-023-38428-2. PMID: 37188688
Cui Z, Napolitano G, de Araujo MEG, Esposito A., Monfregola J, Huber LA, Ballabio A and Hurley JH. Structure of the lysosomal mTORC1-TFEB-Rag-Ragulator mega complex. Nature 2023.
Cattelani C, Lesiak D, Liebscher G, Singer I, Stasyk T, Wallnöfer MH, Heberle AM, Corti C, Hess MW, Pfaller K, Kwiatkowski M, Pramstaller PP, Hicks AA, Thedieck K, Müller T, Huber LA and de Araujo MEG. The SZT2 Interactome Unravels New Functions of the KICSTOR Complex. Cells 2021, 10(10), 2711; PMID:34685691
Napolitano G, Di Malta C, Esposito A, de Araujo MEG, Pece S, Bertalot G, Matarese M, Benedetti V, Zampelli A, Stasyk T, Siciliano D, Venuta A, Cesana M, Vilardo C, Nusco E, Monfregola J, Calcagnì A, Di Fiore PP, Huber LA, Ballabio A. A substrate-specific mTORC1 pathway underlies Birt-Hogg-Dubé syndrome. Nature. 2020 Jul 1. doi: 10.1038/s41586-020-2444-0. PMID:32612235
de Araujo MEG, Liebscher G, Hess MW, Huber LA. Lysosomal size matters. Traffic. 2020 Jan;21(1):60-75. doi: 10.1111/tra.12714. Epub 2019 Dec 6. Review. PMID:31808235
de Araujo MEG, Naschberger A, Fürnrohr BG, Stasyk T, Dunzendorfer-Matt T, Lechner S, Welti S, Kremser L, Shivalingaiah G, Offterdinger M, Lindner HH, Huber LA, Scheffzek K. Crystal structure of the human lysosomal mTORC1 scaffold complex and its impact on signaling. Science. 2017 Sep 21. pii: eaao1583. doi: 10.1126/science.aao1583. PMID: 28935770
Collaborations
Gennaro Napolitano (Tigem, Naples, IT)
David Haselbach (IMP, Vienna, AUT)
Kathrin Thedieck (Institute of Biochemistry,University of Innsbruck, AUT)
Hesso Farhan (Institute of Pathophysiology, Medical University of Innsbruck, AUT)
Funding
FWF FG 2005/61357 – 2023- Organelle proteostasis in cellular quiescence and growth
TWF GZ F16676/5-2019 – A potential new mechanism for selective modulation of mTORC1
Team and Contact

The Araujo team, March 2024 from left Maike, Flora, Mariana, Paula, Thanida
Priv. Dozentin Mariana Eca Guimaraes de Araujo, PhD
Email:mariana.araujo@i-med.ac.at
Phone: +43 512 9003 70174
Google scholar: https://scholar.google.com/citations?hl=en&user=5jH0XooAAAAJ&view_op=list_works&sortby=pubdate
Name
Mariana Eca Guimaraes de Araujo
Thanida Laopanupong
Maike Kämmer
Flora Gradl
Paula Flümann
Position
Group leader
PhD student
Master student – MCI
Master student – Molmed
Master student – Molmed