The Vietor Laboratory: Regulatory Mechanisms of Cell Differentiation
Our group is interested in the roles of the TPA-induced sequence-7 (TIS7, also known as IFRD1) protein and its orthologue SKMc15 (IFRD2). We have shown that they are involved in the regulation of the differentiation of neurons, enterocytes, myocytes and adipocytes and that TIS7 is a transcriptional co-regulator (Lammirato et al., BMC Biol, 2016). We have generated TIS7 and SKMc15 double knockout (dKO) mice and used them to identify a regulatory role for TIS7 in adipocyte differentiation and in resistance to high-fat diet (Vietor et al. eLife, 2023). As dKO mice are substantially leaner, we propose that TIS7 and SKMc15 are involved in physiological adipocyte differentiation. However, our mouse model does not enable us to distinguish between a systemic and a tissue-specific role of the two genes. We are thus generating constitutive knockout mouse models that specifically lack the expression of these genes in either the small intestine or adipocyte tissues. We are also establishing mouse organoid technology to limit the use of experimental animals and to permit us to study the role of the two genes more closely.
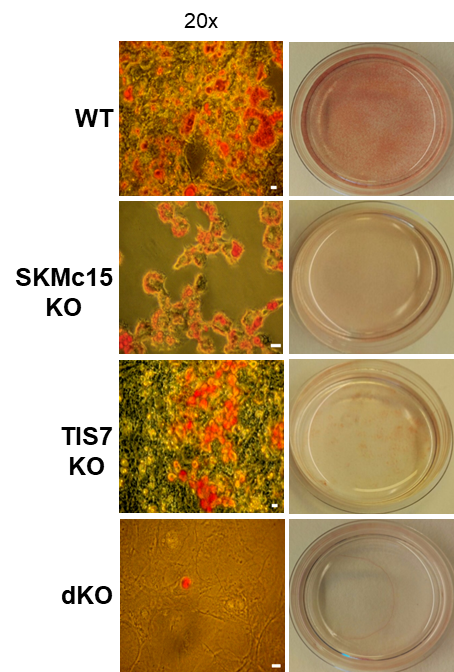
Adipocyte differentiation is regulated by TIS7 and SKMc15. Oil red O-staining of MEF cells isolated from wild-type (WT), TIS7, SKMc15 single knockout and TIS7 SKMc15 double knockout mice after 8 days of the adipocyte differentiation protocol
Publications
An Overview of Autophagy in Hematopoietic Stem Cell Transplantation. Montazersaheb S, Ehsani A, Fathi E, Farahzadi R, Vietor I. Front Bioeng Biotechnol. 2022 May 23;10:849768. doi: 10.3389/fbioe.2022.849768. eCollection 2022. PMID: 35677295.
The negative adipogenesis regulator Dlk1 is transcriptionally regulated by Ifrd1 (TIS7) and translationally by its orthologue Ifrd2 (SKMc15). Vietor I, Cikes D, Piironen K, Vasakou T, Heimdörfer D, Gstir R, Erlacher MD, Tancevski I, Eller P, Demetz E, Hess MW, Kuhn V, Degenhart G, Rozman J, Klingenspor M, Hrabe de Angelis M, Valovka T, Huber LA.Elife. 2023 Aug 21;12:e88350. doi: 10.7554/eLife.88350.PMID: 37603466.
Granulocyte differentiation of rat bone marrow resident C-kit+ hematopoietic stem cells induced by mesenchymal stem cells could be considered as new option in cell-based therapy. Farahzadi R, Fathi E, Mesbah-Namin SA, Vietor I. Regen Ther. 2023 May 3;23:94-101. doi: 10.1016/j.reth.2023.04.004. eCollection 2023 Jun. PMID: 37206538.
Adipose Tissue-Mesenchymal Stem Cells Caused to Change the Methylation Status of hTERT Gene Promoter CpG Islands of Molt-4 Leukemia Cells as Cell-based Therapy. Fathi E, Montazersaheb S, Vandghanooni S, Farahzadi R, Vietor I.Curr Mol Med. 2023;23(3):266-274. doi: 10.2174/1566524022666220118103136.PMID: 35040412
Mesenchymal stem cells cause induction of granulocyte differentiation of rat bone marrow C-kit+ hematopoietic stem cells through JAK3/STAT3, ERK, and PI3K signaling pathways. Fathi E, Mesbah-Namin SA, Vietor I, Farahzadi R.Iran J Basic Med Sci. 2022 Oct;25(10):1222-1227. doi: 10.22038/IJBMS.2022.66737.14633.PMID: 36311196
L-Carnitine Reduced Cellular Aging of Bone Marrow Resident C-Kit+ Hematopoietic Progenitor Cells Through Telomere Dependent Pathways. Fathi E, Montazersaheb S, Sanaat Z, Nakhlband A, Vandghanooni S, Farahzadi R, Vietor I.Curr Stem Cell Res Ther. 2023;18(2):231-236. doi: 10.2174/1574888X17666220511141123.PMID: 35546751
Effect of Rat Bone Marrow Derived-Mesenchymal Stem Cells on Granulocyte Differentiation of Mononuclear Cells as Preclinical Agent in Cellbased Therapy. Fathi E, Azarbad S, Farahzadi R, Javanmardi S, Vietor I.Curr Gene Ther. 2022;22(2):152-161. doi: 10.2174/1566523221666210519111933.PMID: 34011256
Collaborations
Ezzatollah Fathi, Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Tabriz, Tabriz. Iran
Funding
IMPULSE Iran Austria
FWF P36861-B
Contact

Univ. Doz. Dr. Ilja Vietor
Email: ilja.vietor@i-med.ac.at
Phone: +43 512 9003 70175