The Valovka Laboratory: Molecular Basis of Rare Genetic Disorders
Rare diseases are often the result of harmful genetic variants but the precise molecular effects of these variants remain poorly understood. Our group is working to identify the genetic culprits behind the disorders and to uncover how they hijack cellular processes to cause disease. In close collaboration with the Department of Paediatrics and Adolescent Medicine at MUI (Andreas Janecke and Thomas Müller), we have identified and are studying unique pathogenic mutations in various genes, including PCNT, SLC5A1, PCSK1, RIPK1 and UNC45A.
The osteo-oto-hepato-enteric (O2HE) syndrome is an autosomal recessive disease ascribed to loss-of-function mutations in the Unc-45 myosin chaperone A (UNC45A) gene. The clinical spectrum includes bone fragility, hearing loss, cholestasis, neurological symptoms and life-threatening diarrhoea associated with microvillus inclusion disease (MVID)-like enteropathy. We are trying to understand how a deficiency of or specific mutations in UNC45A affect the complex molecular mechanism of this severe neonatal disease. We have described a novel missense variant of UNC45A, c.710T>C, with unique pathogenicity caused by altered chaperone-myosin interactions rather than a complete loss of chaperone function. The findings indicate the possibility of treatment and may guide the development of targeted therapies.
We are also screening natural anti-inflammatory compounds. In collaboration with the Austrian Drug Screening Institute (ADSI), we have developed a screening platform to identify and evaluate bioactive compounds in natural extracts. We were able to show the anti-inflammatory and cytoprotective polypharmacology of the complex herbal medicine Canephron N® (Bionorica SE) and to identify its mechanism of action.
doi: 10.1111/cge.13797
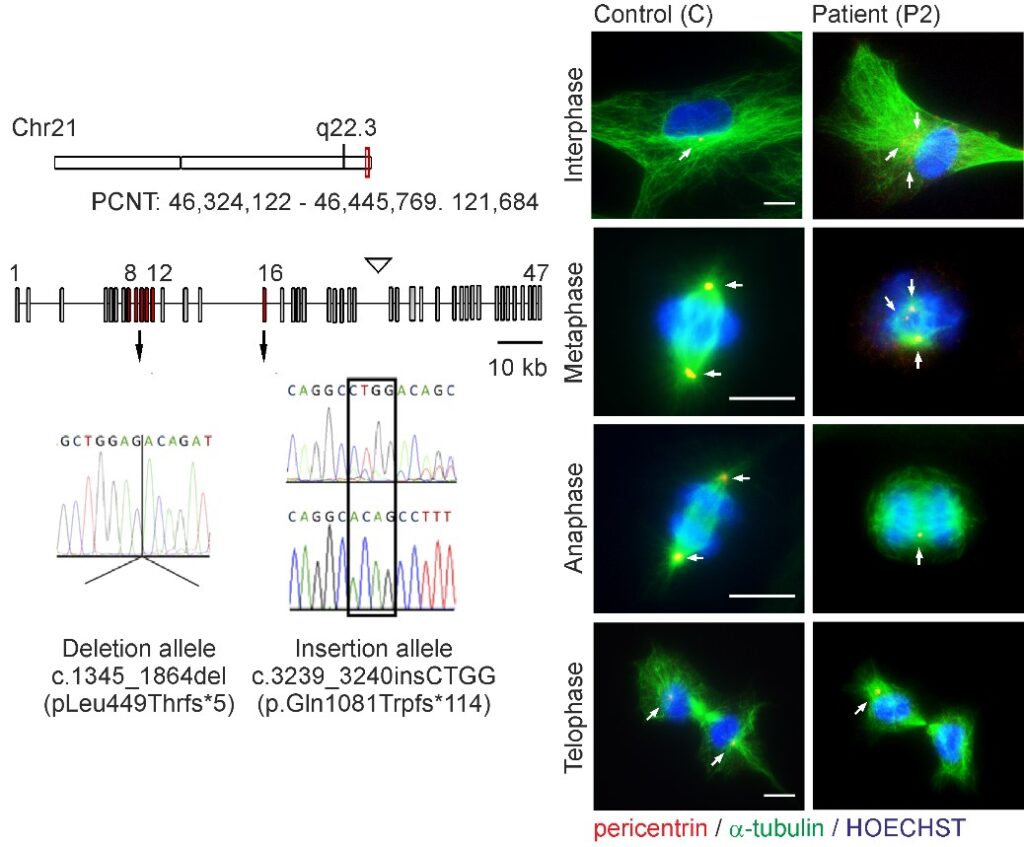
Fig. 5: Abnormal mitotic morphology of MOPDII patient fibroblasts with mutated PCNT variants
Publications
The negative adipogenesis regulator Dlk1 is transcriptionally regulated by Ifrd1 (TIS7) and translationally by its orthologue Ifrd2 (SKMc15). Vietor I, Cikes D, Piironen K, Vasakou T, Heimdörfer D, Gstir R, Erlacher MD, Tancevski I, Eller P, Demetz E, Hess MW, Kuhn V, Degenhart G, Rozman J, Klingenspor M, Hrabe de Angelis M, Valovka T, Huber LA. Elife. 2023 Aug 21;12:e88350. doi: 10.7554/eLife.88350. PMID: 37603466
SLC5A1 Variants in Turkish Patients with Congenital Glucose-Galactose Malabsorption. Hoşnut FÖ, Janecke AR, Şahin G, Vogel GF, Lafcı NG, Bichler P, Müller T, Huber LA, Valovka T, Aksu AÜ. Genes (Basel). 2023 Jun 27;14(7):1359. doi: 10.3390/genes14071359. PMID: 37510265
Novel PCNT variants in MOPDII with attenuated growth restriction and pachygyria. Waich S, Janecke AR, Parson W, Greber-Platzer S, Müller T, Huber LA, Valovka T, Vodopiutz J. Clin Genet. 2020 Sep;98(3):282-287. doi: 10.1111/cge.13797. Epub 2020 Jul 7. PMID: 32557621
Characteristic facial features and cortical blindness distinguish the DOCK7-related epileptic encephalopathy. Haberlandt E, Valovka T, Janjic T, Müller T, Blatsios G, Karall D, Janecke AR. Mol Genet Genomic Med. 2021 Jan 20:e1607. doi: 10.1002/mgg3.1607. Online ahead of print. PMID: 33471954
Fluorescent thermal shift-based method for detection of NF-κB binding to double-stranded DNA. Leitner PD, Vietor I, Huber LA, Valovka T. Sci Rep. 2021 Jan 27;11(1):2331. doi: 10.1038/s41598-021-81743-1.PMID: 33504856
Anti-Inflammatory Extract from Soil Algae Chromochloris zofingiensis Targeting TNFR/NF-κB Signaling at Different Levels. Leitner PD, Jakschitz T, Gstir R, Stuppner S, Perkams S, Kruus M, Trockenbacher A, Griesbeck C, Bonn GK, Huber LA, Valovka T. Cells. 2022 Apr 21;11(9):1407. doi: 10.3390/cells11091407. PMID: 35563717
UNC45A deficiency causes microvillus inclusion disease-like phenotype by impairing myosin VB-dependent apical trafficking. Duclaux-Loras R, Lebreton C, Berthelet J, Charbit-Henrion F, Nicolle O, Revenu de Courtils C, Waich S, Valovka T, Khiat A, Rabant M, Racine C, Guerrera IC, Baptista J, Mahe MM, Hess MW, Durel B, Lefort N, Banal C, Parisot M, Talbotec C, Lacaille F, Ecochard-Dugelay E, Demir AM, Vogel GF, Faivre L, Rodrigues A, Fowler D, Janecke AR, Müller T, Huber LA, Rodrigues-Lima F, Ruemmele FM, Uhlig HH, Del Bene F, Michaux G, Cerf-Bensussan N, Parlato M.J Clin Invest. 2022 May 16;132(10):e154997. doi: 10.1172/JCI154997.PMID: 35575086
Collaborations
The Austrian Drug Screening Institute (ADSI), AUT
Christoph Griesbeck, Department of Biotechnology and Food Engineering, MCI – The Entrepreneurial School, AUT
Julia Vodopiutz, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, AUT
Daniel Kotlarz, Department of Pediatrics, Ludwig-Maximilians-Universität (LMU) Munich, DDSI)
Bionorica SE, DE
Contact

Priv.-Doz. DDr. Taras Valovka
Email: taras.valovka@i-med.ac.at
Phone: 0043 512 9003 70174
